A) Carbocations are stabilized by electron-withdrawing inductive effects only.
B) Carbocations are stabilized by electron-withdrawing inductive effects and hyperconjugation.
C) Carbocations are stabilized by electron-donating inductive effects only.
D) Carbocations are stabilized by electro-donating inductive effects and hyperconjugation.
F) C) and D)
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
Rank the following carbocations in order of decreasing stability, putting the most stable first. 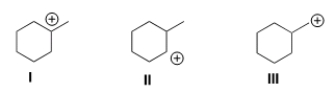
A) I > II > III
B) II > I > III
C) III > I > II
D) III > II > I
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name of the following compound? 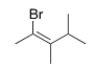
A) (E) -2-Bromo-3,4-dimethyl-2-pentene
B) (Z) -1-Bromo-1,2,3-trimethyl-1-butene
C) (Z) -2-Bromo-3,4-dimethyl-2-pentene
D) (E) -1-Bromo-1,2,3-trimethyl-1-butene
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the product of the nucleophilic substitution reaction shown below? 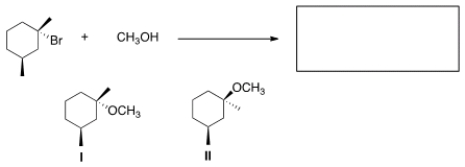
A) Only I
B) Only II
C) I and II
D) None
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about the Hammond postulate is not true?
A) The Hammond postulate provides a quantitative estimate of the energy of a transition state.
B) In endothermic reactions, the transition state is closer in energy to the products.
C) In exothermic reactions, the transition state is closer in energy to the reactants.
D) The Hammond postulate provides a qualitative estimate of the energy of a transition state.
F) A) and D)
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
Which of the following solvents is not a polar protic solvent?
A) I
B) II
C) III
D) IV
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name of the following compound? 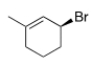
A) (R) -3-Bromo-1-methylcyclohexene
B) (S) -3-Bromo-1-methylcyclohexene
C) (S) -1-Bromo-3-methyl-2-cyclohexene
D) (R) -1-Bromo-3-methyl-2-cyclohexene
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the most nucleophilic?
A) CH4
B) H2O
C) NH3
D) HF
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the following substitution reaction, what would the effect be of changing the solvent from CH3OH to (CH3) 2S=O? CH3(CH2) 5Br + NaOH CH3(CH2) 5OH + Br-
A) The rate would decrease because SN1 reactions are favored by polar protic solvents.
B) The rate would increase because SN2 reactions are favored by polar aprotic solvents.
C) The rate would increase because SN1 reactions are favored by polar protic solvents.
D) The rate would decrease because SN2 reactions are favored by polar aprotic solvents.
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reaction of bromoethane with sodium acetate affords the substitution product methyl acetate. What is the effect of doubling the concentration of sodium acetate on the rate of the reaction?
A) The rate remains the same.
B) The rate decreases by a factor of 2.
C) The rate increases by a factor of 2.
D) The rate increases by a factor of 4.
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name of the following compound? 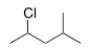
A) 2-Methyl-4-chloropentane
B) 2-Chloro-4-methylpentane
C) 2-Chloro-1-isopropylpropane
D) 2-Chloro-2-methylpentane
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following structures have the correct common name? 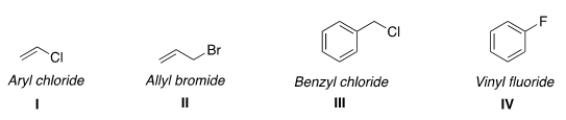
A) I and II
B) II and IV
C) II and III
D) III and IV
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which compound is most likely to follow second-order kinetics in a substitution reaction?
A) CH3Br
B) (CH3) 3CCH2Br
C) CH3CH2Br
D) (CH3) 2CHBr
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following structures have the correct common name? 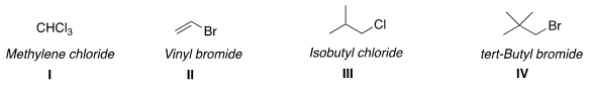
A) I and II
B) II and III
C) I and III
D) III and IV
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about SN2 reactions is true?
A) The rate of reaction is dependent on just the substrate.
B) The fastest reaction will occur with a tertiary alkyl halide.
C) The mechanism is a two-step process.
D) Displacement occurs with inversion of configuration.
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the product of the nucleophilic substitution reaction shown below? 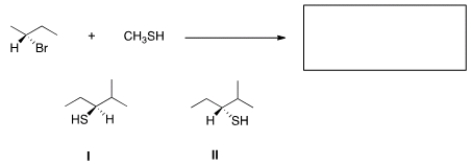
A) Only I
B) Only II
C) I and II
D) None
F) A) and B)
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
What is the product of the nucleophilic substitution reaction shown below? 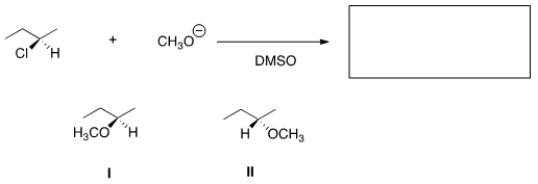
A) Only I
B) Only II
C) I and II
D) None
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the product of the nucleophilic substitution reaction shown below? 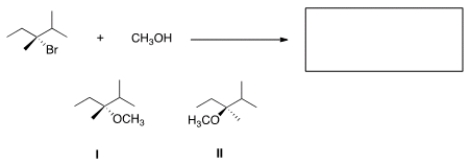
A) Only I
B) Only II
C) I and II
D) None
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following alkyl halides will react fastest with CH3OH in an SN1 mechanism? 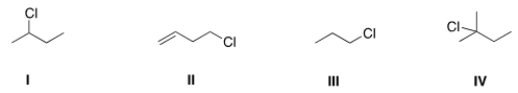
A) I
B) II
C) III
D) IV
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name of the following compound? 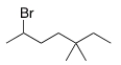
A) 2-Bromo-5,5-dimethylheptane
B) 3,3-Dimethyl-6-bromoheptane
C) 6-Bromo-3,3-dimethylheptane
D) 2-Bromo-5,5-dimethyloctane
F) All of the above
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 64
Related Exams