B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select a statement that best describes the oxidation process.
A) In the oxidation process all elements change oxidation state.
B) In the oxidation process all elements experience an increase in oxidation state.
C) In the oxidation process some elements change their oxidation state.
D) In the oxidation process some elements experience oxidation state increase.
E) In the oxidation process only oxygen increases its oxidation state.
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which of the following pairs is the oxidation number for the underlined element INCORRECT?
A) ClO4-/(+7)
B) S2O32-/(+2)
C) Fe2O3/(+3)
D) HCO3-/(+3)
E) CO2/(+4)
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many millilitres of 0.550 mol L-1 hydriodic acid are needed to react with 15.00 mL of 0.217 mol L-1 CsOH? HI(aq) + CsOH(aq) → CsI(aq) + H2O(l)
A) 0.0263 mL
B) 0.169 mL
C) 5.92 mL
D) 38.0 mL
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What concentration of Fe2(SO4) 3(aq) is required for the solution to have [Fe3+(aq) ] = 0.32 M?
A) 0.16 M
B) 0.32 M
C) 0.64 M
D) 0.080 M
E) 0.12 M
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many electrons are transferred when the perchlorate ion is converted to chloride?
A) 8
B) 7
C) 9
D) 6
E) 5
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The substance BaCl2 is a:
A) salt
B) weak acid
C) strong base
D) weak base
E) strong acid
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is an Arrhenius base?
A) CH3OH
B) CH3CO2H
C) HOCl
D) CsOH
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Balance the following oxidation-reduction reaction in acid solution: Fe(s) → Fe2+ + H2(g) The sum of the coefficients is ________.
A) 2
B) 5
C) 8
D) 7
E) 9
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The substance KClO4 in water solution is a:
A) strong electrolyte
B) nonelectrolyte
C) weak electrolyte
D) strong base
E) weak acid
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is quite insoluble in water,but would liberate a gas when treated with aqueous acetic acid?
A) K2CO3
B) K2SO4
C) CaCO3
D) CaSO4
E) AgCl
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The substance NH3 is a:
A) weak acid
B) strong acid
C) salt
D) weak base
E) strong base
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The neutralization of 25.0 mL of 0.24 M HCl requires 5.0 mL of NaOH.What is the molarity of the NaOH solution?
A) 0.6 M
B) 2.4 M
C) 5.0 M
D) 0.048 M
E) 1.2 M
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Indicate whether a precipitate forms by completing equation Na+(aq) + Cl-(aq) + NO3-(aq) + K+(aq) → ?
A) NaCl(s) + NO3-(aq) + K+(aq)
B) NaNO3(s) + K+(aq) + Cl-(aq)
C) KCl(s) + Na+(aq) + NO3-(aq)
D) KNO3(s) + Na+(aq) + Cl-(aq)
E) no reaction
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In an acid-base neutralization reaction,38.74 mL of 0.500 mol L-1 potassium hydroxide reacts with 50.00 mL of sulfuric acid solution.What is the concentration of the H2SO4 solution?
A) 0.194 mol L-1
B) 0.387 mol L-1
C) 0.775 mol L-1
D) 1.29 mol L-1
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to the balanced equation shown below,4.00 moles of oxalic acid,H2C2O4,reacts with ________ moles of permanganate,MnO4-. 5 H2C2O4(aq) + 2 MnO4-(aq) + 6 H+(aq) → 10 CO2(g) + Mn2+(aq) + 8 H2O(l)
A) 1.60
B) 4.00
C) 8.00
D) 9.00
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following,in aqueous solution,is most likely to behave as a weak acid?
A) HCl
B) HCN
C) NaCl
D) NaCN
E) NH3
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Balance the following equation in basic solution: Br2 + Mn2+ → MnO2 + Br- The sum of the coefficients is ________.
A) 8
B) 15
C) 12
D) 17
E) 11
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Lead ions can be precipitated from aqueous solutions by the addition of aqueous iodide: 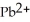 (aq) +
(aq) + 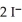 (aq) →
(aq) → 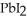 (s)
Lead iodide is virtually insoluble in water so that the reaction appears to go to completion.How many millilitres of 3.550 mol L-1 HI(aq) must be added to a solution containing 0.700 mol of
(s)
Lead iodide is virtually insoluble in water so that the reaction appears to go to completion.How many millilitres of 3.550 mol L-1 HI(aq) must be added to a solution containing 0.700 mol of 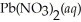 To completely precipitate the lead?
To completely precipitate the lead?
A) 2.54 × 10-3 mL
B) 394 mL
C) 197 mL
D) 0.197 mL
E) 0.394 mL
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following pairs of aqueous solutions will give a precipitate when mixed?
A) K3PO4 and CaCl2
B) LiClO4 and Ba(OH) 2
C) AgNO3 and Ca(ClO4) 2
D) NaCl and K(CH3COO)
E) Sr(NO3) 2 and KI
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Showing 81 - 100 of 124
Related Exams