A) I
B) II
C) III
D) IV
F) A) and D)
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
How many liters of hydrogen gas are needed to produce 100.0 grams of water at 25.0°C and 1.00 atm pressure according to the chemical equation shown below? 2 H2(g) + O2(g) → 2 H2O(l)
A) 67.9 L
B) 136 L
C) 203 L
D) 407 L
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many liters of oxygen gas can be produced at STP from the decomposition of 0.250 L of 3.00 M H2O2 in the reaction according to the chemical equation shown below? 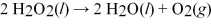
A) 8) 41 L
B) 11.2 L
C) 16.8 L
D) 33.6 L
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many liters of hydrogen gas can be generated by reacting 9.25 grams of barium hydride with water at 20°C and 755 mm Hg pressure according to the chemical equation shown below? BaH2(s) + 2 H2O(l) → Ba(OH) 2(aq) + 2 H2(g)
A) 0) 219 L
B) 0) 799 L
C) 1) 60 L
D) 3) 21 L
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many grams of KCl(s) are produced from the thermal decomposition of KClO3(s) which produces 50.0 mL of O2(g) at 25°C and 1.00 atm pressure according to the chemical equation shown below? 2 KClO3(s) → 2 KCl(s) + 3 O2(g)
A) 0) 102 g
B) 0) 152 g
C) 0) 167 g
D) 0) 304 g
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following compounds is a hydrate?
A) H2O2
B) H2SO4
C) CoCl2 ∙ 6 H2O
D) Cu(OH) 2
F) B) and C)
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
What is not a characteristic of ozone,O3?
A) It is a nonlinear triatomic system.
B) It has one single bond and one double bond.
C) It is an extremely powerful oxidizing agent.
D) It is made by passing an electrical discharge through oxygen.
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is classified as an amphoteric binary oxide?
A) B2O3
B) CO2
C) Al2O3
D) SiO2
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 7.63-gram sample of hydrated magnesium sulfate MgSO4∙ xH2O is heated until all of the water is driven off.If 3.72 grams of anhydrous MgSO4 is obtained,how many water molecules combine with each formula unit of magnesium sulfate in the hydrate?
A) 5
B) 6
C) 7
D) 8
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many liters of hydrogen gas are needed to produce 90.0 grams of water at 25.0°C and 1.00 atm pressure according to the chemical equation shown below? 2 H2(g) + O2(g) → 2 H2O(l)
A) 61.1 L
B) 122 L
C) 183 L
D) 366 L
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which isotope of diatomic hydrogen should have the highest heat of vaporization?
A) H2
B) D2
C) T2
D) The values are equal.
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If the molar mass of monoatomic deuterium (D) is 2.0141 grams then what is the density of diatomic deuterium (D2) gas at 25.0°C and 1.00 atmosphere pressure?
A) 0) 082 g/L
B) 0) 165 g/L
C) 0) 329 g/L
D) 12.2 g/L
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
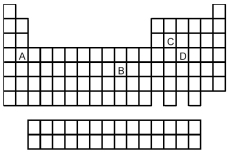 -Which element shown on the periodic table above forms a covalent binary hydride with the formula MX3?
-Which element shown on the periodic table above forms a covalent binary hydride with the formula MX3?
A) element A
B) element B
C) element C
D) element D
F) All of the above
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
Look at the location of elements A,B,C,and D in the following periodic table.Consider the oxides that form when the elements A-D are in their highest oxidation states. 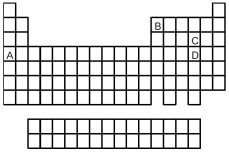 -Which oxide is the most covalent?
-Which oxide is the most covalent?
A) A
B) B
C) C
D) D
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the preparation of oxygen by the thermal decomposition of potassium chlorate shown below,what is the oxidation number change of the atom undergoing reduction? 2 KClO3(s) → 2 KCl(s) + 3 O2(g)
A) - 8
B) - 6
C) + 1
D) + 2
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Compounds that absorb water from the atmosphere are said to be
A) hygroscopic.
B) macroscopic.
C) orthoscopic.
D) symbiotic.
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many liters of O2 gas at 25°C and 1.00 atm pressure are needed to react with 60.25 grams of potassium hydride according to the chemical equation shown below? 2 KH(s) + O2(g) → H2O(l) + K2O(s)
A) 9) 19 L
B) 18.4 L
C) 36.8 L
D) 73.5 L
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which has the greatest number of unpaired electrons?
A) O2-
B) O2
C) O2-
D) O22-
F) All of the above
Correct Answer

verified
Correct Answer
verified
Short Answer
Superoxide ion,O2-,peroxide ion,O22-,and oxide ion,O2-,contain ________,________,and ________ unpaired electrons,respectively.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
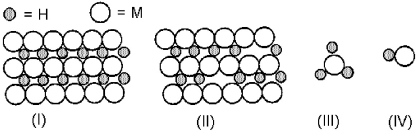 -Using the above models of hydrides,indicate the one most representative of an ionic hydride.
-Using the above models of hydrides,indicate the one most representative of an ionic hydride.
A) I
B) II
C) III
D) IV
F) None of the above
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 175
Related Exams