A) α decay.
B) β decay.
C) ![]() decay.
decay.
D) electron capture.
E) spontaneous fission.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the nuclide that completes the following nuclear reaction. 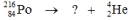
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the nuclide that completes the following nuclear reaction. 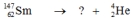
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the missing species in the following nuclear transmutation. 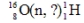
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following types of radioactive decay does not produce new element?
A) gamma emission
B) electron capture
C) beta emission
D) alpha emission
E) double beta emission
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the missing species in the following nuclear transmutation. 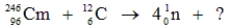
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) None of these choices are correct.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The isotope 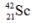 is unstable. This is predictable because
is unstable. This is predictable because
A) the number of neutrons is too large in relation to the number of protons.
B) the number of neutrons is too small in relation to the number of protons.
C) the atomic number is too large.
D) the mass number is too large.
E) Sc isotopes are all unstable.
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following cannot be an example of nuclear fusion?
A) A nuclear reaction in which two reactants have greater binding energy than their fused product.
B) A nuclear reaction in which a carbon nucleus is produced.
C) The primary energy-producing reaction in the Sun.
D) The primary energy-producing reaction in a hydrogen bomb.
E) A reaction in which the reactants are less stable than the products.
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
So-called "magic numbers" of particles are thought to convey extra stability to certain nuclei. These magic numbers refer to which of the following particles?
A) protons only
B) electrons only
C) positrons only
D) neutrons only
E) protons and neutrons
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Carbon-14 will emit a β particle with an energy of 0.1565 MeV. What is this energy in joules?
A) 1.0 × 10-24 J
B) 2.5 × 10-20 J
C) 1.0 × 10-18 J
D) 2.5 × 10-14 J
E) None of these choices are correct.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following equations correctly represents electron capture by the 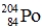 nucleus?
nucleus?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following elements is formed largely in supernova explosions?
A) H
B) He
C) Mg
D) Fe
E) U
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 9.52 × 10-5 mol sample of rubidium-86 emits 8.87 × 1016 β particles in one hour. What is the half-life of rubidium-86?
A) 2.23 × 10-3 h
B) 1.55 × 10-3 h
C) 448 h
D) 645 h
E) None of these choices are correct.
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A patient's thyroid gland is to be exposed to an average of 5.5 μCi for 16 days as an ingested sample of iodine-131 decays. If the energy of the β radiation is 9.7 × 10-14 J and the mass of the thyroid is 32.0 g, what is the dose received by the patient?
A) 0.027 rads
B) 1.2 rads
C) 37 rads
D) 85 rads
E) None of these choices are correct.
G) D) and E)
Correct Answer

verified
Correct Answer
verified
True/False
Radioactive decay follows zero-order kinetics.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The radiochemist, Will I. Glow, studied thorium-232 and found that 2.82 × 10-7 moles emitted 8.42 × 106 α particles in one year. What is the decay constant for thorium-232?
A) 3.35 × 10-14 yr-1
B) 4.96 × 10-11 yr-1
C) 1.40 × 1010 yr-1
D) 2.99 × 1013 yr-1
E) None of these choices are correct.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
True/False
Positron decay and electron capture have the same net effect on the Z and N values of a nucleus.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Iodine-131, t1/2 = 8.0 days, is used in diagnosis and treatment of thyroid gland diseases. If a laboratory sample of iodine-131 initially emits 9.95 × 1018 β particles per day, how long will it take for the activity to drop to 6.22 × 1017 β particles per day?
A) 2.0 days
B) 16 days
C) 32 days
D) 128 days
E) None of these choices are correct.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 7.85 × 10-5 mol sample of copper-61 emits 1.47 × 1019 positrons in 90.0 minutes. What is the decay constant for copper-61?
A) 0.00230 h-1
B) 0.00346 h-1
C) 0.207 h-1
D) 0.311 h-1
E) None of these choices are correct.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Sodium-21 will emit positrons each having an energy of 4.0 × 10-13 J. What is this energy in MeV?
A) 4.0 × 10-7 MeV
B) 2.5 MeV
C) 40 MeV
D) 2.5 × 106 MeV
E) None of these choices are correct.
G) None of the above
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 81
Related Exams